адитивността на една такава енергетична характеристика е задължителна, за да има смисъл всяко едно количествено сравнение на нейните изменения от молекула в молекула, както и нейната преносимост в набор от подобни молекули. В полето MMX тя се постига чрез специалния вид на полето: сума от множество членове на полето по структурните елементи - химични връзки, валентни ъгли, диедрични ъгли, както и по двойките несвързани атоми за Ван-дер-Валсовите взаимодействия.
stretch - опъвам, протягам се (английски)
bend - огъвам, прегъвам (английски)
torsion - изкривяване, извиване (английски)
в 1-4 положение са, например азотният и кислородният атом в 2-аминоетанола, H2NCH2CH2OH. Кислородният атом и двата въглеродни се намират, съответно в 1-2 и 1-3 положение.
конфигурация на молекулата - пространственото разположение на атомите в молекулата. В по-тесен смисъл се разбира разположението на атомите един към друг, еднозначно определено само от дължините на връзките и валентните ъгли. По-долу е дадено определението в Encyclopedia Britanica:
Configuration in chemistry, the spatial arrangement of atoms in a molecule. The configuration is usually depicted by means of a three-dimensional model (a ball-and-stick model), a perspective drawing, or a plane projection diagram.
Until late in the 20th century, the experimental determination of absolute or actual configuration (i.e., the true three-dimensional form of the molecule) was a difficult process; therefore, there were few substances with known absolute configurations (e.g., tartaric acid). Many configurations were, for convenience, assigned by correlation with glyceraldehyde, for which the following configurations (as represented by plane projection diagrams) have been determined:
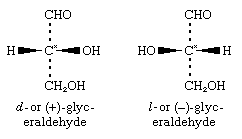
The configuration of d-glyceraldehyde, in which the hydroxyl group is attached to the right of the asymmetric carbon centre (starred in the formulas), is designated as D, and the configuration of l-glyceraldehyde, in which the hydroxyl group is to the left of the asymmetric carbon, is designated as L. Thus, the complete designation of d-glyceraldehyde is given by D-d-glyceraldehyde (D to specify configuration and d to specify the direction of optical rotation); and that of l-glyceraldehyde, L-l-glyceraldehyde.
Today, optical and chemical methods make it possible to determine the absolute configuration of practically any molecule. In the most modern scheme for specifying absolute configurations, D-d-glyceraldehyde is designated (R)-(+)-glyceraldehyde, and L-l-glyceraldehyde is (S)-(-)-glyceraldehyde. The letters R and S denote the absolute configurations at the asymmetric carbon atoms according to a set of rules that assign priorities to the four attached atoms or groups, and the + and - signs indicate the directions of the optical rotations.
Compounds are then assigned D or
L configurations on the basis of their genetic relationship with the appropriate
form of glyceraldehyde. In the imagined transformation of glyceraldehyde
to the substance whose configuration is in question, it is assumed that
none of the steps involved causes any change in the configuration at the
asymmetric carbon. For compounds containing several asymmetric carbon atoms,
it is essential to specify which carbon atom was compared with glyceraldehyde.
When the R-S system is used, the absolute configuration must be stated
for each asymmetric carbon atom in the molecule.
конформация на молекулата - всяко едно пространствено разположение на атомите в пространството, което се получава при завъртане около единична връзка на една част от молекулата спрямо останалата. Възможни са безкраен брой такива разположения на атомите в молекулата. По-долу е дадено определението в Encyclopedia Britanica:
Conformation in chemistry - any one of the infinite number of possible spatial arrangements of atoms in a molecule that result from rotation of its constituent groups of atoms about single bonds.
Different conformations are possible
for any molecule in which a single covalent bond connects two polyatomic
groups, in each of which at least one atom does not lie along the axis
of the single bond in question. The simplest such molecule is that of hydrogen
peroxide, in which the two hydroxyl groups can rotate with respect to one
another about the axis of the oxygen-oxygen bond. The presence of more
than one such single bond in a molecule--as in that of propane (CH3
-CH2-CH3), for example--merely adds to the complexity
of the situation without changing its nature. In molecules such as those
of cyanogen (N![]() C-C
C-C![]() N)
or butadiyne (H-C
N)
or butadiyne (H-C![]() C-C
C-C![]() C-H),
all the atoms lie along the axis of the central single bond, so that no
distinguishable conformations exist.
C-H),
all the atoms lie along the axis of the central single bond, so that no
distinguishable conformations exist.
In general, every distinguishable conformation of a molecule represents a state of different potential energy because of the operation of attractive or repulsive forces that vary with the distances between different parts of the structure. If these forces were absent, all conformations would have the same energy, and rotation about the single bond would be completely free or unrestricted. If the forces are strong, different conformations differ greatly in energy or stability: the molecule will ordinarily occupy a stable state (one of low energy) and undergo a transition to another stable state only upon absorbing enough energy to reach and pass through the unstable intervening conformation.
The intramolecular forces in ethane, for example, are so weak that their existence can be inferred only from subtle effects on thermodynamic properties such as enthalpy and entropy. (Even if internal rotation in ethane were severely restricted, its three most stable conformations are indistinguishable.) The molecular structures of certain more complex compounds, however, impose such strong barriers to rotation that stereoisomeric forms--differing only in conformation--are stable enough to be isolated.
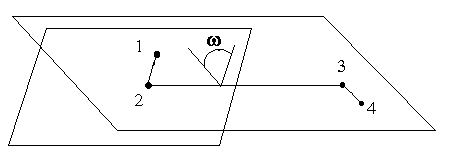
диедричен ъгъл (dihedral angle) е ъгълът между двете равнини, образувани от четири атома, свързани с поне три връзки. Първата равнина е образувана от първите три от атомите, а втората - от последните три. При тази дефиниция се предполага, че трите атома в тези две тройки не лежат на една права. На следната фигура е показан диедречният ъгъл, w, между атомите 1-2-3-4.
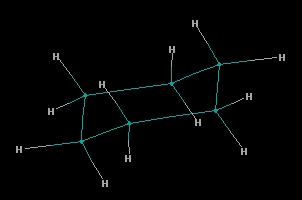
Конформацията "стол" на циклохексана, изчислена с програмата PCModel